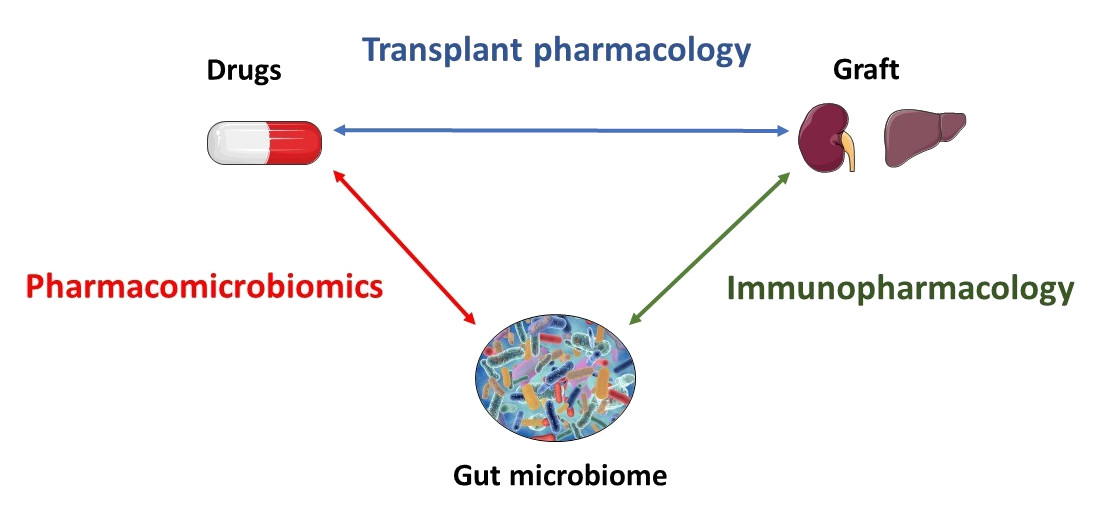
Our unit is dedicated to precision medicine in transplantation by merging multi-scale knowledge and research on immunosuppressive drugs. In this perspective, our team investigates gut microbiota-host-immunosuppressant drug interplay and its contribution to the interindividual variability of transplant outcomes. Therefore, we are therefore conducting several projects in the field of pharmacomicrobiomics and immunopharmacology.

Roland Lawson
Project manager
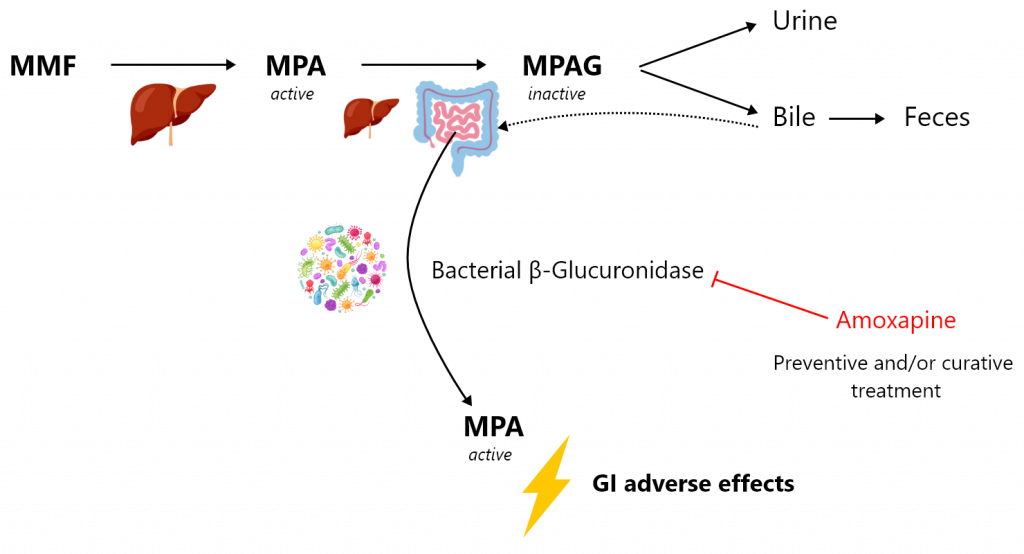
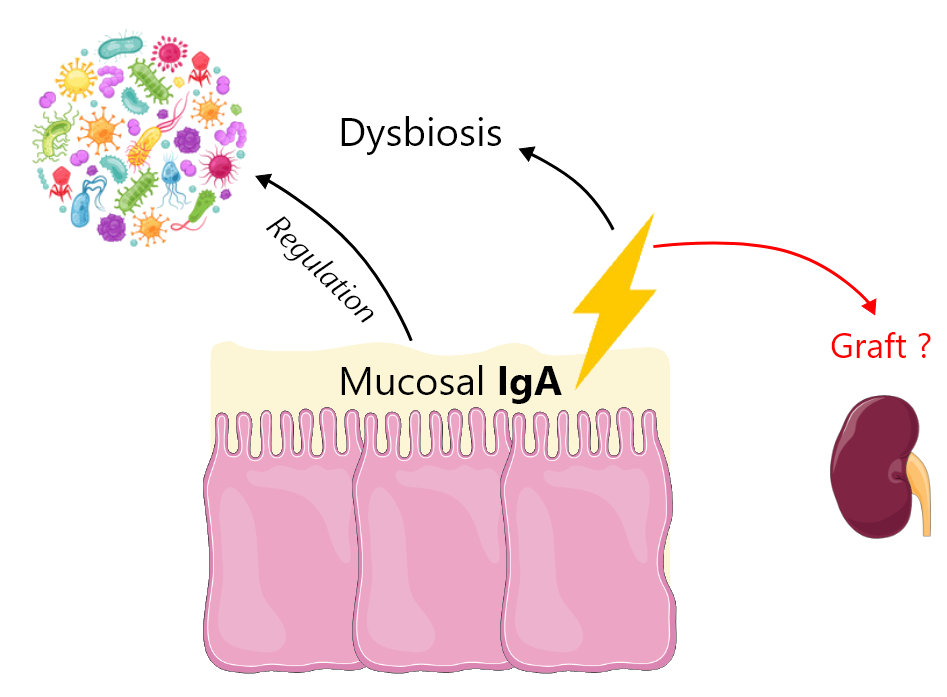
Mycophenolate mofetil (MMF) is one of the most effective immunosuppressants. It is widely used in solid organ and bone marrow transplantation as well as in autoimmune disorders (e.g., lupus erythematosus, rheumatoid arthritis, psoriatic arthritis or vasculitis). MMF treatment is unfortunately associated with multiple adverse effects, among which the gastrointestinal effects are of major concern. More than 30% of patients complain of gastrointestinal adverse effects, which can range from simple nausea, vomiting, abdominal pain or diarrhoea to bleeding ulcerations and erosions of the gastrointestinal tract. They can occur regardless of MMF exposure duration, leading to a significant decrease of patient adherence to the treatment. These gastrointestinal adverse effects of MMF are likely to be mediated by the intestinal back-transformation of the metabolite mycophenolic acid glucuronide (MPAG) excreted by the hepatocytes in bile, into the active parent form mycophenolic acid (MPA). Bacterial β-glucuronidase (β-G) expressed by certain bacterial phyla found in the microbiome are able to transform MPAG into MPA. Thus, a change in the intestinal microbiota or intestinal dysbiosis in transplanted patients, which may favour the proliferation of bacteria expressing this enzyme or induce its expression, are likely to result in increased concentrations of MPA in the distal intestine, resulting in turn in gastrointestinal adverse effects.
Our project aims to evaluate the effect of the targeted inhibition of bacterial β-G as a preventive and/or curative treatment of MPA-induced gastrointestinal damage. We will evaluate, in a mouse MMF-induced colitis model, the therapeutic potential of a selective bacterial β-G inhibitor, amoxapine. Amoxapine is an antidepressant used in human that is also a potent inhibitor of bacterial β-G with no antibiotic effect. In parallel, we will characterize new candidate inhibitors of bacterial β-G by means of high-throughput screening of a large academic chemical library. This approach will be supported by atomic-scale thermodynamic modelling of β-G inhibition.