Biomarkers
Non-invasive, early, and specific biomarkers could prove very useful for the effective management of chronic liver diseases and for preventing complications related to liver or kidney transplants. Preventing liver and kidney diseases is the best strategy to avoid transplantation. Therefore, the use of early diagnostic or predictive strategies is crucial to quickly identify affected patients for better clinical management, to reduce the morbidity and mortality associated with these widespread diseases, and to avoid or delay the need for transplantation.
In kidney transplantation, the P&T team is researching urinary biomarkers for graft rejection, ischemia-reperfusion, drug-induced nephrotoxicity, and proximal tubular dysfunction. These various projects are inherently multidisciplinary and therefore aim to develop new diagnostic and prognostic approaches to improve the clinical management of patients with chronic liver or kidney diseases, as well as transplant recipients, paving the way for more targeted and personalized therapeutic interventions
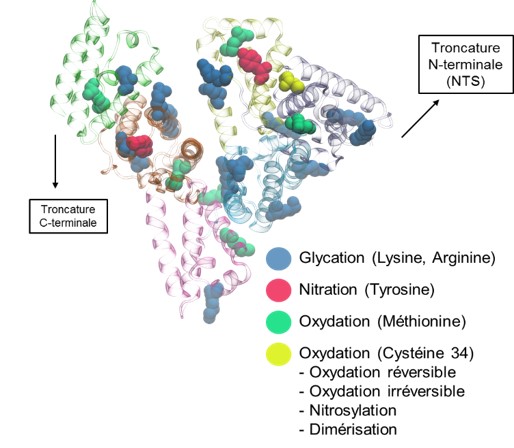
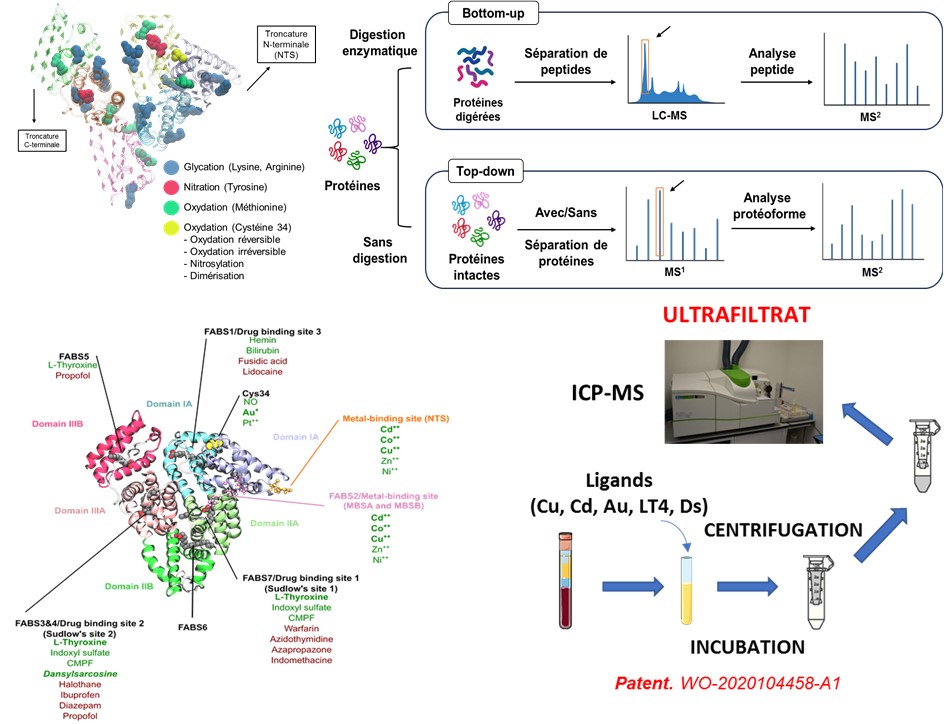
Our research explores the detection and quantification of specific albumin isoforms as indicators of liver damage. We have developed the “Serum Enhanced Binding” (SEB) test, which detects structural changes in albumin through ligand-binding models measured by mass spectrometry. In preclinical research, this method has successfully detected liver dysfunction at early stages.
Our project relies on several approaches: analytical chemistry to characterize albumin modifications, ex vivo studies on hepatocyte cell lines to understand the mechanisms of damage, preclinical models to monitor the appearance of PTMs, clinical studies to assess the predictive value of these biomarkers, in silico simulations to analyze binding behaviors, and data analysis through machine learning to link these biomarkers to the nature and severity of the disease. Our goal is to improve the diagnosis of liver diseases and complications of liver transplants, paving the way for more precise care.
The primary goal of this project is to explore the performance of post-translational modifications (PTMs) of albumin (HSA) as a very early, simple, and reliable biomarker for the onset of EAD, thereby facilitating the rapid management of patients with liver transplant failure. HSA is produced exclusively by the liver, and its post-translational modifications (glycosylations and others) appear to differ in liver damage. Therefore, post-translational modifications of HSA could serve as valuable indicators of the condition of the transplanted liver in the days following transplantation. Using advanced high-resolution mass spectrometry techniques (LC-HR-MS), the variations and distribution of HSA isoforms will allow us to identify patients at risk of graft rejection and thus improve the prediction and management of post-transplant complications. The SEB test described above will also be evaluated for this purpose and adapted if necessary.
The influx and efflux membrane transporters of the proximal renal tubules regulate the active excretion and reabsorption of many endogenous and drug molecules. The team has previously demonstrated the vulnerability of these transporters to ischemia-reperfusion injury related to transplantation. As part of scientific collaborations within and outside the FHU SUPORT, this research is evolving towards the search for specific endogenous biomarkers of tubular transport to more precisely assess proximal tubular damage, with early applications in the management of post-transplant complications.