Development of ‘smart’ metal-matrix or metal-dopant antibacterial surface coatings by dry deposition techniques Développement de surfaces ‘intelligentes’ antibactériennes par voie sèche suivant deux approches ‘matrice métal’ et ‘dopant métal’
Laurène YOUSSEF ,
Audrey PROROT ,
Alain DENOIRJEAN ,
Laurène GNODÉ ,
Thibault MAERTEN
et Canet ACIKGOZ
The emergence of the SARS-CoV-2 virus in late 2019 shook the scientific community. Research teams from different fields then quickly mobilized to seek adequate and efficient long-term solutions. However, pathogens are not only limited to viruses: bacteria are microorganisms found in all environments and also likely to cause human diseases. One of the possible transmission routes is through contact with surfaces touched by infected people. In this work, coatings with an antibacterial matrix of Cu are developed by plasma spraying at IRCER, Limoges and doped with a photo-catalyst, TiO2, in order to evaluate the synergy of the two effects on the bactericidal character of Cu. In parallel, TiN coatings with antibacterial dopants (Ag and Cu) elaborated by PVD, are supplied by Oerlikon Balzers, Liechtenstein. All antibacterial tests are carried out at E2Lim, Limoges with the aim of comparing on the one hand the effect of the quantity of a bactericidal element and on the other hand the surface condition of the coating on its ability to destroy pathogens. Results of studies by flow cytometry of the bacteria’s enzymatic activity are presented in this work.
L’émergence du virus SARS-CoV-2 fin 2019 a secoué la communauté scientifique. Des équipes de recherche de différents domaines se sont alors rapidement mobilisées pour chercher des solutions adéquates et efficaces à long terme. Les agents pathogènes ne sont cependant pas limités aux virus : des bactéries, microorganismes présents dans tous les milieux sont aussi susceptibles de causer des maladies chez les humains. Une des voies de transmission possibles est le contact avec des surfaces touchées par des personnes infectées. Dans ce travail, des revêtements à matrice antibactérienne de Cu sont élaborées par projection plasma à l’IRCER, Limoges et dopées avec un photo-catalyseur, le TiO2, afin d’évaluer la synergie des deux effets sur le caractère bactéricide du Cu. En parallèle, des revêtements de TiN à dopants antibactériens (Ag et Cu) élaborés par PVD, sont fournis par Oerlikon Balzers, Liechtenstein. Tous les tests antibactériens sont effectués à E2Lim, Limoges avec l’objectif de comparer d’une part l’effet de la quantité d’un élément bactéricide et d’une autre part l’état de surface du revêtement sur sa capacité à détruire les agents pathogènes. Quelques resultats d’étude par cytométrie à flux de l’activité enzymatique de la bactérie sont présentés dans ce travail.
‘In the longer run and for wide-reaching issues, more creative solutions tend to come from imaginative interdisciplinary collaboration’
Robert J. Shiller
1. Introduction
Following the emergence of the COVID-19 pandemic in early 2020, efforts in academic research have been doubled in order to offer affordable protection solutions against pathogens. However, pathogens are not limited to viruses. Indeed, bacteria, microorganisms present in all environments, are also likely to cause human diseases. Knowing that these bacteria are ubiquitous especially on surfaces [1], it makes sense to get interested in materials qualified as 'multifunctional' reducing the spread of these microorganisms, as well as decomposing organic and inorganic pollutants using light as an activation source for example [2]. All this with the aim of maintaining a healthy environment, thus meeting the increasingly strict demands of today's society. To return to the subject of materials with antimicrobial potential, copper (Cu) is the oldest metal known to human civilization. Its use dates back to around the 5th millennium B.C. The first medical applications of Cu were mentioned in Smith Papyrus, one of the oldest books in history [3]. On the other hand, certain reviews published in recent years discuss the antibacterial potential of 2D materials [4], biomaterials [5] as well as noble metals such as silver (Ag), gold (Au) and platinum (Pt) [6].
These works present metallic elements as the most promising in terms of antimicrobial and fungicidal efficacy. However, in their massive state, these elements remain relatively expensive and toxic in vivo and in vitro if present in large quantities. Indeed, Cu is an essential 'trace' element in living organisms and participates in the composition of more than 30 proteins known today such as tyrosinase (melanin synthesis), lysyl oxidase (collagen cross-linking) or cytochrome C oxidase involved in the regulation of the respiratory chain [7]. As for silver, at cumulative doses of around 70 to 150 mg.kg-1 of body weight, it is likely to cause dermatoses as well as renal, hepatic or neurological failure [8].
To go around this limitation, scientists have implemented processes capable of producing high-quality surface coverings with controlled metal quantities using thermal [9] and cold [10] plasmas. Indeed, the development of such surfaces being in full expansion, the choice of the dry method, in particular plasma spray (PS) and physical vapor deposition (PVD), is based on the ability to control the structure, the microstructure, the texture, composition and consequently, the different properties of the materials. The choice is also based on the feasibility of this type of multifunctional coating on various metallic substrates such as steel, and heat-sensitive ones such as glass, wood or plastic. In addition, through these techniques, some compounds could be added to the initial material in order to improve its antibacterial efficiency or even give it some interesting new properties. Consequently, the purpose of the study is the comparison of the efficacies of an 'antibacterial Cu matrix' doped by a photo-catalytic compound (TiO2) and a biocompatible and mechanically solid matrix (TiN), doped by antibacterial elements like Cu or Ag. These materials are supposed to be used as surface coverings of daily used tools or objects in public transports (bus, train, plane), medical supplies or pipelines for water distribution. The project consortium is presented in Appendix 1.
2. Main objectives
This study sets up three main objectives: (1) monitoring the photo-catalytic activity of metal matrix (Cu) materials doped with TiO2 obtained by plasma spray (PS), (2) evaluating the antibacterial efficacy of these coatings before and after doping and that of a PVD-titanium nitride matrix (TiN) doped with Ag and Cu, and (3) studying membrane integrity and enzymatic activity of the bacteria in order to understand the mechanism of antimicrobial action.
3. Literature review
According to previous and recent studies, several materials have demonstrated remarkable antimicrobial properties. As mentioned in the introduction, the event that draw the scientific community to the subject was the emergence of the SARS-CoV-2 virus at the end of 2019. Since then, researchers have been developing innovative solutions every day to fight microorganisms. Indeed, these are not limited to viruses but there are also bacteria, algae and fungi. Thus, finding a potential material to combat all or a maximum of microorganisms present on the surfaces at once, is necessary especially for medical utensils and public use. In addition, these materials could be used to coat the inside of pipes, for example to combat micropollutants in water. The common antibacterial materials belong to ceramic families, epoxy resins, and metals such as silver, copper, zinc, gold and platinum [6,11]. Indeed, antibacterial surfaces are effective against a wide variety of bacteria. They often contain active ingredients based on silver or copper, allowing effective sanitation, but they provide more advanced protection by continuously inhibiting the growth of microbes (viruses, bacteria, fungi, algae) for longer durations [11]. Scientists are particularly interested in silver and copper for a variety of reasons explained in the following part.
Silver
This metal, with germicidal and bactericidal properties, was already used in the form of plates since antiquity to purify water, thus providing drinkable water [12]. Along with gold, platinum and palladium, silver is one of the four precious metals. Known since antiquity, its name comes from the Latin Argentum, or the Indian Sanskrit Ar-jun which means “shining”. Until today, this metal, highly coveted despite the fluctuation of its cost, has always been used in different forms in everyday life. Pure silver is bright white, and has high electrical and thermal conductivities, which constitutes an advantage for the development of plasma coatings as explained by Boyer et al. [13]. At bacteria level, reactions on the surface of a silver nanoparticle have the possibility of weakening the membrane of the bacteria leading to its destruction (Figure 1).
Figure 1: Schematic representation of the main possible reactions on a silver nanoparticle surface [13].
Silver nanoparticles can neutralize the charge of the membrane by adsorption and thus contribute to its weakening. This causes leakage of the cytoplasm (Figure 1). Once the membrane is pierced, the nanoparticles can enter the bacteria through the formed pores. These nanoparticles can also enter through ‘porins’, proteins of the bacterial membrane allowing the exchange of ions or hydrophilic molecules [13]. These nanoparticles can also interact with the DNA and condense it, making its replication impossible [13].
Copper
The name of this metal is originated from Latin Cuprum which designates 'the Romans who imported copper from Cyprus'. It is the first metal to be used by man since 5000 B.C. and the third most used metal in the world after iron and aluminum. This era, called the Copper Age, allowed the establishment of metallurgy for decorations, jewelry, tableware, and silverware. Nowadays, this metal, abundant in certain areas of the Earth, appears in various forms. There is gray copper associated with antimony and arsenic, copper sulfide, copper in the form of oxides (cupric ions Cu2+ or copper ions Cu+) and copper in the form of deposits. The first applications of this metal in medicine are mentioned in the book Smith Papyrus [3] and it is ideal for developing antibacterial surfaces [14].
Copper’s mechanism of action on microorganisms is still debated in the scientific community. Bacterial death due to copper exposure could be caused by: depolarization of the bacterial membrane, destruction of the bacterial envelope, attack of bacterial DNA, or redox reactions [14]. These different mechanisms of action of Cu on the bacteria are shown schematically in Figure 2.
Figure 2: Copper action mechanisms on bacteria via (A) Surface Cu, (B) Cellular lysis, (C) Reactive Oxygen Species (ROS) and (D) DNA destruction [7].
Despite its antibacterial properties, copper becomes toxic if present in large quantities in the human body. It is a trace element essential to life in the sense that it helps in the production of blood hemoglobin, it strengthens the immune system and it participates in the constitution of some proteins [7]. However, in case of absorption of large doses of copper or one of its derivatives, life-threatening intoxication manifested by violent vomiting occurs. In addition, Cu2+ ions present in the body can react with thiols (RSH) following the reaction [7]: 2Cu2+ + 2RSH → 2Cu+ + RSSR + 2H+.
The Cu+ ions thus react with the H+ ions and the following O2: 2Cu+ + 2H+ + O2 → 2Cu2+ + H2O2 and then with the hydrogen peroxide generated, Cu+ + H2O2 → Cu2+ + OH- + OH* [7]. In these three reactions, H2O2 is generated and causes the formation of hydroxyl OH* radicals. The presence of these two compounds in large quantities would cause cellular death rather than bacterial death.
Supporting and additive elements
As discussed above, the development of pure silver or copper coating is very expensive or even unnecessary since the main reactions occur on the surface. For this, it is essential to find a matrix that supports antibacterial dopants.
The required properties of such matrices are mechanical strength and resistance to scratches and abrasion. Furthermore, since the antibacterial dopants will be deposited by the PVD process, it would be interesting to find a matrix that can be obtained using the same technique and has the required properties. The nitrides and carbides of transition metals, commonly Ti, Ni, Ta, V, Ag, Pt and Hf, are interesting for their hardness, corrosion resistance, endurance and good mechanical properties [15]. Furthermore, these compounds exhibit a rate of ductility and plastic deformation thanks to their metallic sublattices. Nevertheless, titanium nitride (TiN) represents 90% of the coatings market and its study dates back to the early 1980s [15]. It has exceptional mechanical properties which allows its use on tools as an anti-wear layer, or as a diffusion barrier in integrated circuits. Therefore, this material is very interesting to combine with antibacterial materials with the aim of hardening daily contact surfaces and disinfecting them, simultaneously.
Some compounds could be combined to the antibacterial elements (Ag or Cu) to enhance the oxidation/reduction surface reactions. Titanium dioxide (TiO2) is one of the best candidates since it could be activated by UV light. In fact, light absorption with sufficient energy causes the transition of an electron from the valence band to the conduction band creating electron-hole pairs. TiO2 is then no longer insulating but its conductivity is governed by the movement of these electron-hole pairs. Thus, reduction sites are created by the freed electrons, while the holes will constitute sites for oxidation reactions. These surface redox phenomena are very interesting for many applications such as water treatment, self-cleaning glasses, microbiological activity and energy generation [16]. The photocatalytic effect applied to kill bacteria is presented in Figure 3.
Figure 3: Schematic representation of the bacteria damage by photocatalytic effect [17].
Reactive oxygen species, especially the hydroxyl groups OH* (Figure 3), are very efficient at damaging bacteria membranes, allowing other species to enter the cell, causing its death. Finally, three main TiO2 polymorphs have been identified in the literature: anatase, rutile and brookite [18]. Brookite is the least studied and has not demonstrated remarkable photocatalytic activity compared to anatase and rutile, mainly due to the high defect concentration in its structure. Meanwhile, rutile and anatase have both a tetragonal symmetry, anatase being the low temperature (~400 °C) metastable phase form, as it transforms into rutile when heated up to 650-700°C. Thus, the thermodynamically stable phase is rutile.
As a result, TiO2 has maximum activity in the UV range (355 to 380 nm), corresponding to the gap between the valence and the conduction bands (3-3.2 eV). In photocatalysis, the phase with higher efficiency is anatase. This efficiency is due to a longer lifetime of the electron-hole pairs in this phase since the gap is defined as 'indirect', that is to say additional energy is necessary to bring the electron back to its ground state [16].
4. Main results obtained as part of the RESIST project
The copper based ‘metal matrix’ coatings (Cu:TiO2) were elaborated by plasma spray (PS) on stainless steel substrates (SS). Sandblasting, using Corindon (Al2O3, F36 425-600 µm) was performed before the deposition to clean the surface and to enhance coating adherence. The Cu powder is spherical shaped with a nominal particle size distribution -90+38 µm from MetcoTM whereas the TiO2 powder is of Titanium (IV) oxide 99.0-100.5%, AnalaR NORMAPUR® anatase/rutile’ from VMR ChemicalsTM (max. 60 nm particle size). During the plasma spraying process, Cu is supplied in powder whereas TiO2 is injected in a water-based suspension due its extremely small particle size. The purpose is to conserve the maximum amount of photocatalytic anatase at the surface since the temperature could reach above 10 000 K during the elaboration process. In parallel, titanium nitride coatings (TiN) doped with silver (Ag) and copper (Cu) were elaborated by physical vapor deposition (PVD) technique and supplied by Oerlikon BalzersTM, Liechtenstein. The substrates are polished medical grade stainless steel since the growth mechanism in PVD ensures good coating adherence without sandblasting. The photos of the different samples are grouped in Figure 4.
Figure 4: Photos of the PVD and PS samples elaborated and tested in this work.
From a macroscopic point of you (Figure 4), it could be noticed that the PVD coatings are shinier than the PS coatings which implies different roughness level, in consequence a different wettability by the bacterial solution during the antibacterial tests. Scanning Electron Microscopy (SEM) was then performed on the surface to confirm this observation (Figure 5).
Figure 5: SEM micrographs of (a) Cu/SS, (b) Cu:TiO2/SS, (c) TiN:Ag/polished SS and (d) TiN:Cu/polished SS.
The macroscopic observation is confirmed in Figure 5. Both PS Cu and Cu:TiO2 (Figure 5 (a) and (b)) present a rougher aspect compared to the PVD TiN based coatings (Figure 5 (c) and (d)). This result is logic since the substrate surface state is very different (sandblasted in PS and polished in PVD) according to the process mechanism and requirements. However, in Figure 5 (c) and (d), numerous surface dots are visible. X-Ray Dispersive Energy (EDX) confirms that it is neither Ag, nor Cu from doping but more Ti. In fact, droplets are often generated in the cathodic arc PVD process due to local heating of the Ti target. Thus, the Ti droplets originating from this target, pit and develop micro-particles that drop out immediately after the coating process is finished as reported by Ali et al. [19].
The mean atomic percentages detected on three-surface points by EDX in the TiN coatings are 6±0.2 at.% and 5.6±0.6 at.% for TiN:Ag and TiN:Cu, respectively. This shows that the PVD coatings are approximatively at the same doping level into the TiN matrix.
As for the Cu:TiO2 films, EDX shows a Ti/O atomic ratio of 0.53 indicating that the stoichiometric TiO2 was properly incorporated in the matrix. The results also showed an incorporation up to 16 wt.% of TiO2 in copper.
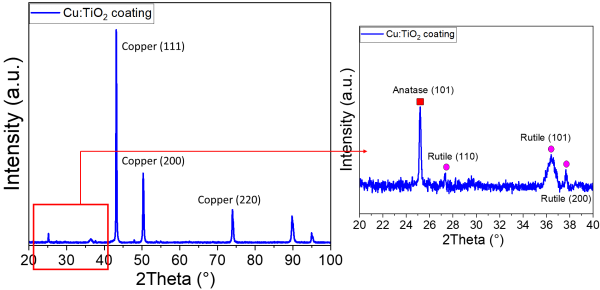
As explained previously, TiO2 anatase polymorph has demonstrated the best photocatalytic activity. However, the challenge in PS is to conserve the maximum amount of this phase because, due to the process high temperature, the incorporated powder will tend to transform into rutile. For this, the X-Ray diffractogram of the Cu:TiO2 film is presented in Figure 6.
Figure 6: XRD diffractogram of the Cu:TiO2 coating on SS.
Figure 6 shows that TiO2 is present in the Cu matrix. It is normal that the relative intensities of TiO2 peaks are very weak compared to those of Cu, because Cu constitutes approximatively 85% of the coating total weight. Despite the process high temperature, the anatase (A) phase is well conserved (peak at 2Theta~25°). The high temperature phase (rutile, R) is also present, which is logic. The anatase fraction could be calculated according to the formula: %A = [8IA / (8IA + 13IR)]*100 [20], I being the peak intensity of anatase (101) and rutile (110). According to the formula, the remaining anatase fraction in the coating reaches 77%, which is not very far from the anatase percentage in the initial powder (~82%). This is probably due to the preparation into a water suspension and the cooling during the plasma process, that does not allow the substrate surface temperature to exceed 360 °C.
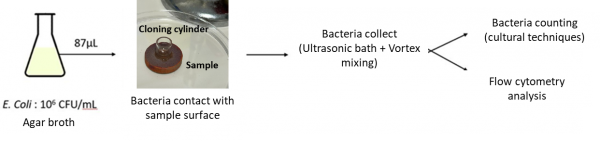
As for the antibacterial tests, they were performed at E2Lim laboratory in Limoges. The summary of the main steps is presented in Figure 7.
Figure 7: Schematic summary of the main steps in the antibacterial tests performed at E2Lim.
In this study, Escherichia Coli is used (CFU=Colony Forming Unit), a Gram-negative bacterium that is surrounded by an outer membrane containing lipopolysaccharide [21]. According to the Centers for Disease Control and Prevention, E. Coli are able to find new ways to be resistant, even to multiple drugs and most available antibiotics [22]. A volume of 87 µL, containing 106 CFU was deposited on the sample surface then the bacteria were collected using vortex mixing and ultrasonic bath after 1h contact with the surface.
As explained in Figures 4 and 5, the surface state of the PS and PVD samples are not the same. Moreover, the sample sizes are different. For this, in order to ensure the same contact surface with bacterial solution, a cloning cylinder have been used according to the recommendation of Oerlikon Balzers based on the ASTM 2180 standard [23].
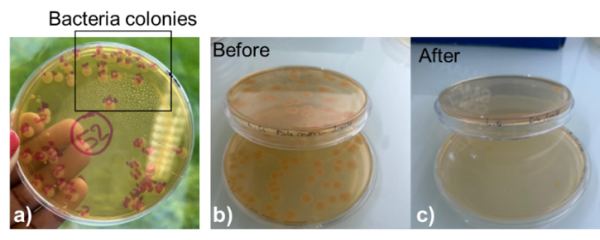
As presented in Figure 7, a part of the bacteria collected from the surface undergoes culture techniques and bacteria counting. By that, the bacteria colonies are counted after contact with the surface as shown in Figure 8 (a).
Figure 8: Photos of (a) an example of bacteria colonies obtained using culture techniques, (b) bacteria colonies before contact with a sample, (c) bacteria colonies after contact with Cu/SS surface.
As given in the example of Figure 8 (b), the bacteria initial concentration was around 106 CFU.mL-1 before contact (CFU is the unit to estimate the number of viable bacteria on a sample surface). After one-hour exposure to a Cu surface on stainless steel, the concentration drops down to 4.102 CFU.mL-1 and on Figure 8 (c), almost no colonies could be observed. By that, a bacterial ‘log reduction’ could be calculated according to the following formula:
Log reduction = Log10 (initial CFU/final CFU) [24]
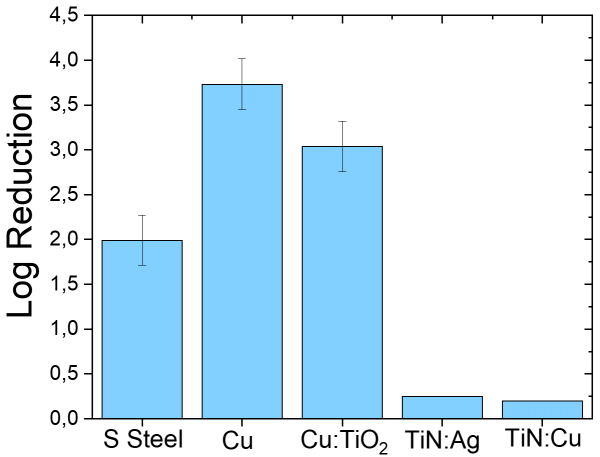
For example, 1Log reduction means a 90% (one ‘9’) bacteria elimination, the ‘decontamination’ threshold is thus fixed at minimum 2Log and the ‘disinfection’ at minimum 4Log reduction. The bacterial log reduction after 1h contact on the different samples is summarized in Figure 9.
Figure 9: Bacterial log reduction obtained on the different surfaces after 1h exposure.
It can be observed that bacteria elimination is enhanced by around 50% when the stainless-steel surface is covered with Cu. On the latter surface, the log reduction exceeds the decontamination (2Log) thus reaching the disinfection threshold (near 4Log). However, when TiO2 is added to the matrix (by ~16 wt.% as mentioned previously), the log reduction slightly decreases. It is important to mention that the antibacterial test shown in Figure 9 is performed without illumination, that may be efficient to activate TiO2 and enhance bacteria elimination. The detail of the photocatalytic tests will not be given in this article because the results are in progress but a preliminary result showed that the Cu:TiO2 sample exhibited near 3% degradation of methylene blue after 100 min UV irradiation (365 nm). This percentage is relatively lower than the results with only TiO2 but it is still explicable by the fact that Cu, that is not photocatalytic, constitutes the major part of the matrix. In addition, a non-negligible amount of TiO2 might be incorporated in the Cu bulk instead of being exposed to the light on the surface. Though, the antibacterial test under light irradiation should be performed because 3% degradation is still an indication that redox reactions might help bacteria elimination.
As for the results on the smooth TiN:Ag and TiN:Cu surfaces, bacteria elimination does not exceed 1Log reduction which is very low. However, this does not indicate that these surfaces are not efficient. In fact, the bacteria solution has been only exposed 1h to the PVD coatings whereas the ASTM 2180 standard indicates a minimum exposure time of 24h. Since the TiN based coatings are smoother than the PS coatings, the bacterial degradation kinetics might be slower, so the bacterial solution should be exposed for a longer time. In addition, the protocol should also be adapted according to the sample surface state. In any case, the antibacterial tests on TiN-based samples should be repeated and longer exposure times tested.
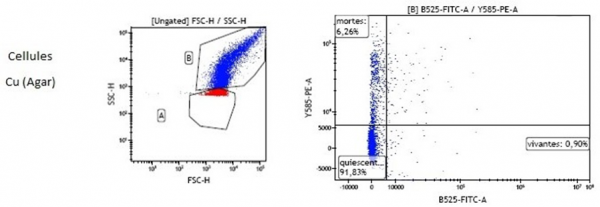
Finally, as shown in Figure 7, the second part of the collected bacteria was analyzed by Flow Cytometry (CMF). The principle of the technique is detailed in Appendix 2. After contact with the coated surface, the cells are marked using two fluorescent substances: Propidium Iodide (PI) and 5(6)-Carboxy-Fluorescein Di-Acetate (CFDA). PI diffuses into damaged membranes and irreversibly intercalates at the level of the cell's DNA, which produces red fluorescence detected by the standard flow cytometer (FL-3 photomultiplier). On the other hand, CFDA serves as a compound that spreads in cell membranes and hydrolyzes by intrinsic esterase activity which leads to green fluorescence measured by CMF using an excitation laser and a photomultiplier (FL-1). An example performed on Cu is presented in Figure 10.
Figure 10: Cytograms obtained by CMF after 1h cell exposure to copper surface.
The results obtained in Figure 10 show that only 6% of the bacterial population would be positive for PI (red marking), and would therefore be permeabilized, after one hour of contact with copper. This percentage is very low in comparison with the data obtained by culture techniques that show near 4Log after 1h exposure to Cu (Figure 9). Additional tests were therefore carried out to ensure that the marking conditions were optimal. The same marking protocol was carried out on untreated cells (control) in a Phosphate Buffered Saline solution (PBS) and in an agar broth identical to that used to deposit the cells on the coatings. It is probable that agar, which is a mixture of two unbranched polysaccharide components, disrupts cell marking by inhibiting the penetration of markers inside the cells and modifies the fluidics of CMF analysis. The results are still under investigation.
5. Conclusion
This work is based on the development of antibacterial coatings using two dry deposition techniques: Plasma Spray (PS) and Physical Vapor Deposition (PVD).
The PS process, carried out at IRCER laboratory, used sandblasted stainless steel on which copper (Cu) has been deposited. In order to evaluate the antibacterial effect enhancement by light, a photosensitive material (TiO2) is added to the Cu matrix (Cu:TiO2). The SEM observations showed a relatively rough surface and the EDX revealed a TiO2 incorporation up to 16 wt.% into the Cu matrix. However, the preliminary photocatalytic test showed only 3% methylene blue degradation after 100 min irradiation by UV light (365 nm). This low photocatalytic response might be due to the fact that the major percentage of TiO2 is more incorporated into the copper matrix than exposed to the surface, where the redox reactions should happen. In parallel, TiN-based coatings were deposited by PVD technique on medical grade polished stainless steel and supplied by Oerlikon Balzers. The antibacterial doping agents were Ag and Cu. The SEM images showed a relatively smooth surface with some particles mainly due to the droplet effect of the cathodic arc evaporation process. The TiN matrix has been chosen for its excellent mechanical properties and its biocompatibility. The EDX analysis showed an average doping level of 6 at.% of both Ag and Cu.
The antibacterial tests have been performed at E2Lim laboratory. Escherischia Coli (E. Coli) has been used as a model to simulate the biological surface contamination. A volume of 87 µL, containing 106 CFU was deposited on the sample surface. The bacterial solution deposition took place according to the ASTM 2180 standard recommended by Oerlikon Balzers. In fact, in order to ensure the same contact surface, cloning cylinders were used during the inoculation. The bacteria were collected using vortex mixing and ultrasonic bath after 1h contact with the surface. A part of this bacteria were counted using the cultural technique (bacteria colonies counting). On Cu, the bacterial reduction log reached the disinfection threshold (4Log) whereas on Cu:TiO2 the reduction log was near 3Log. Since the sample had not been irradiated, it is logic that Cu:TiO2 showed a lower activity than pure Cu since it has less antibacterial agent (Cu). Even though the photodegradation percentage was low, the antibacterial test under UV irradiation on Cu:TiO2 sample is still worth the investigation to see if the bacteria elimination efficiency is enhanced compared to pure Cu. As for the bacterial reduction on TiN:Ag and TiN:Cu, the results did not exceed 0.5Log which corresponds to very low efficacy. In fact, the standard recommends 24h exposure time on such surfaces since they are smooth, so the degradation kinetics might be slower than that on rough samples. Moreover, the solution adhesion on smooth surfaces is not similar to that on the rough ones, due to the different surface tension (hydrophilic and hydrophobic caracter). For this, an inverstigation of the contact angle is mandatory on each surface state.
Finally, CMF analysis showed that only 6% of the bacterial population would be permeabilized, after one hour of contact with Cu. This percentage is very low compared to the bacterial reduction log obtained by culture techniques. It is probable that agar disrupted cell marking by inhibiting the markers penetration inside the cells and modified the fluidics of CMF analysis. Technical studies are ongoing to validate the photocatalytic tests, repeat the results on TiN-based coatings by the standards, perform antibacterial tests under illumination and improve the CMF analysis protocol, for surface disinfection and water treatment applications.
![Figure 1: Schematic representation of the main possible reactions on a silver nanoparticle surface [13].](docannexe/image/751/img-1-small600.png)
![Figure 2: Copper action mechanisms on bacteria via (A) Surface Cu, (B) Cellular lysis, (C) Reactive Oxygen Species (ROS) and (D) DNA destruction [7].](docannexe/image/751/img-2-small600.png)
![Figure 3: Schematic representation of the bacteria damage by photocatalytic effect [17].](docannexe/image/751/img-3.png)