The classical (power) Freundlich adsorption isotherm is defined by the following equation:
where q is the adsorbed quantity, c the concentration
of the adsorbate in solution, KF and α are parameters.
In the case of gas adsorption, c is replaced by the pressure p.
Recent research has shown that this equation is an approximation of
a more general equation: the hypergeometric Freundlich isotherm:
|
q = qm |
x
1 + x
|
·F ( 1, 1, α + 1 ; |
1
1 + x
|
) |
|
where qm is the maximal value of q, x = c / c° or p / p°,
c° and p° being the reference concentration or pressure. F is the
Gauss hypergeometric function.
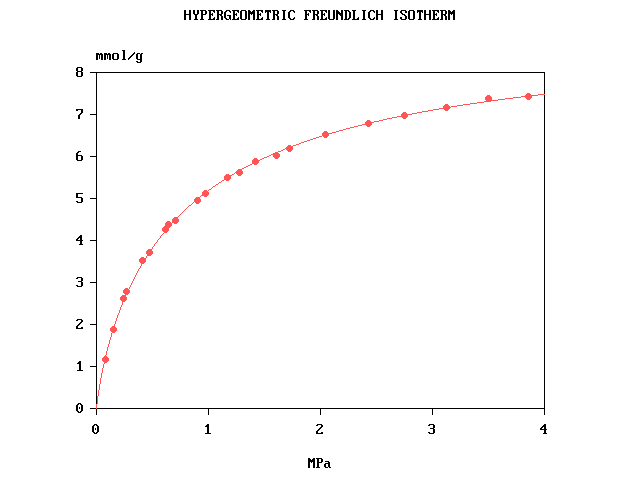
Fitting the hypergeometric Freundlich isotherm
The computer program Freundlich.bas written in FreeBASIC fits the curve by nonlinear
regression, using simulated annealing followed by Marquardt's method. The fitted parameters are c° (or p°), qm and α.
Reference
J. Debord, K. H. Chu, M. Harel, S. Salvestrini, J.-C. Bollinger.
Yesterday, Today and Tomorrow. Evolution of a Sleeping Beauty: The Freundlich Isotherm.
Langmuir, 2023, 39, 3062–3071. https://doi.org/10.1021/acs.langmuir.2c03105

