Clinical Research
The Clinical Research Unit in Pharmacology and Toxicology (RCPT), located within the Department of Pharmacology, Toxicology, and Pharmacovigilance at the University Hospital of Limoges (CHU de Limoges), serves as a support structure for UMR-1248 Pharmacology & Transplantation.
It coordinates clinical studies sponsored by CHU de Limoges and INSERM, working closely with other units within the department (Biological Pharmacology and Pharmacokinetics, Pharmacometrics and Artificial Intelligence, and Environmental Toxicology and Occupational Health) as well as clinical departments involved in the projects (notably Nephrology, Dialysis and Transplantation, and Hepato-Gastroenterology and Nutrition). These studies primarily focus on immunosuppressive drugs, used in transplantation as well as in other conditions such as autoimmune diseases and cancer.

Caroline Monchaud
Responsible
The RCPT Clinical Research Unit supports members of UMR1248 in designing clinical research projects, collaborating with the sponsor throughout the entire process—from the initial research idea to its valorization. This includes: search for funding, literature review, formulating research hypotheses, regulatory compliance, drafting clinical research protocols, coordinating participating centers, managing biological samples, conducting pharmacology and toxicology analyses, data management, statistical analyses and pharmacokinetic modeling, preparing final study reports, presenting findings at conferences and publishing in scientific journals.
The RCPT Unit constitutes and manages biological collections related to the research themes of UMR1248. It is a member of CRBioLim (Biological Resource Center of CHU Limoges) and is responsible for storing biological resources (biological samples and associated data recorded in specialized software) in accordance with current standards. Its key focus areas include: “biomarkers in transplantation”, “autoimmune diseases”, “oncology biomarkers” and “biomarkers of response to anti-infective treatments”.
The RCPT Unit’s activities comply with Good Clinical Practices (GCP) and current regulations. Additionally, it collaborates closely with INSERM UMR1248 research and CRBioLim operations for biological sample management.
Consequently, RCPT’s activities align with the laboratory’s quality systems, which are accredited under the ISO 15189 medical biology standard and certified under the ISO 20387 biobank standard (CRBioLim).
Some examples
EASY is a clinical drug study titled “Evaluation of the Benefits of Administering Immunosuppressive Drugs as Single Daily Doses Over the First Year After Liver Transplantation”.
The primary objective is to evaluate the benefits of a once-daily immunosuppressive regimen during the first 18 months following liver transplantation.
The study also employs an innovative volumetric absorptive microsampling strategy developed by Trajan, allowing patients to autonomously perform at-home blood collections needed for tacrolimus pharmacological monitoring.
The study plans to recruit 200 patients across nearly all liver transplantation centers in France.
EASY is sponsored by CHU de Limoges and receives financial support from CHIESI Laboratories and Trajan.
The study is coordinated by Dr. Marilyne Debette-Gratien, hepatologist, and Dr. Caroline Monchaud, pharmacologist, both at CHU de Limoges.
ClinicalTrial number: NCT06354179
Recruitment phase ongoing as of fall 2025.
For more information:
| Tryptique de l’étude | Newletter N°1 décembre 2024 | Newletter N°2 mai 2025 | FAQ |
MALAHBAR is a clinical study titled “Albumin Modifications as Early Biomarkers of Chronic Liver Diseases (MALAHBAR)”.
The primary objective is to evaluate the ability of albumin post-translational modifications to predict the progression of liver disease over a 3-year period in patients with advanced chronic liver disease (ACLD), based on the occurrence of liver-related events or the worsening of disease stage as assessed by the Child-Pugh and MELD scores.
The study aims to recruit 756 patients across 7 university hospitals (CHUs) in France.
MALAHBAR is sponsored by CHU de Limoges and funded by the 2022 Interregional Hospital Clinical Research Program (PHRC interrégional 2022).
The study is coordinated by Dr. Paul Carrier, hepatologist, and Dr. Souleiman El Balkhi, pharmacologist.
ClinicalTrial number: NCT06318949
Recruitment phase ongoing as of fall 2025.
For more information:
| Tryptique de l’étude | Newletter N°1 mars 2025 | Newletter N°2 juillet 2025 |
REXETRIS is a data-based study titled “Long-term Exposure–Effect Relationships in Renal Transplant Recipients Treated with Immunosuppressive Drugs.”
The primary objective is to investigate the relationships between prolonged exposure to immunosuppressive drugs in kidney transplant recipients and the long-term outcomes of both the patient and the graft.
The REXETRIS database results from the integration of three data sources: ABIS (CHU de Limoges), CRISTAL (French Biomedicine Agency), and SNDS (French National Health Data System, CNAM).
It includes data from 47,842 French kidney transplant recipients transplanted between 2005 and 2021.
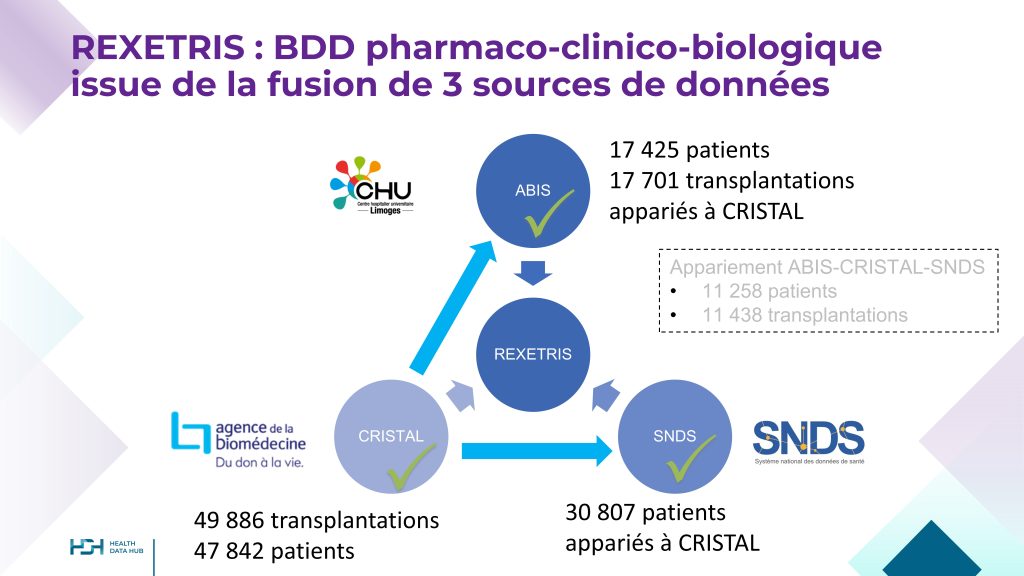
REXETRIS, winner of the first Health Data Hub call for projects in 2019, is led by Prof. Marquet and managed by the University Hospital of Limoges (CHU de Limoges).
Statistical analyses are ongoing as of fall 2025.
https://www.health-data-hub.fr/partenariats/rexetris
Short video: https://www.youtube.com/watch?v=zwbTIRCBMrc
Full video: https://www.youtube.com/watch?v=54zXVVRdndg
S. Couderc, S. Crépin, M. Labriffe et al., Dealing with large packaging in algorithms using the French health reimbursement data system (SNDS) database, Therapies, https://doi.org/10.1016/j.therap.2025.02.001
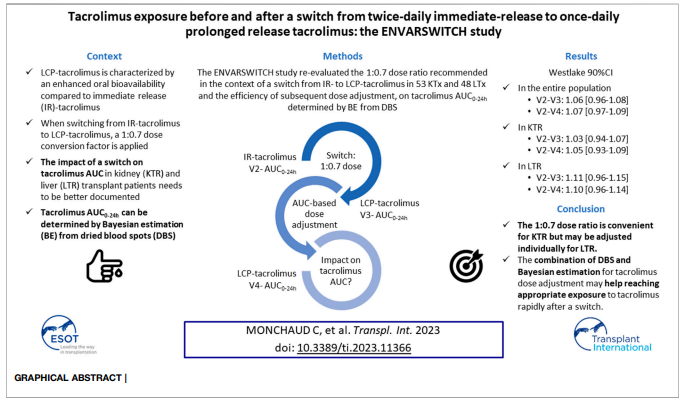
ENVARSWITCH is a clinical study titled “PK Assessment of Tacrolimus Exposure Before and After a Switch From Twice Daily Immediate-release (Prograf®) to Once-daily Prolonged Release Tacrolimus (Envarsus®)”.
The primary objective was to verify the absence of difference between the pre- and post-conversion tacrolimus AUC₀₋₂₄h, calculated using Bayesian estimation, in kidney or liver transplant patients converted from Prograf® to Envarsus® at a 1:0.7 dose ratio, followed by dose adjustment to match the pre-conversion AUC₀₋₂₄h target.
The study implemented a dried blood spot (DBS) microsampling strategy, allowing patients to independently and conveniently perform at-home blood collections required for tacrolimus therapeutic drug monitoring.
A total of 134 patients (70 kidney and 64 liver transplant recipients) were enrolled across 17 transplantation centers in France.
ENVARSWITCH was sponsored by CHU de Limoges and received financial support from CHIESI Laboratories.
The project, led by Prof. Pierre Marquet, was coordinated by Dr. Caroline Monchaud.
ClinicalTrial number: NCT02882828
Study results were published in: Monchaud C, Woillard JB, Crépin S, et al. Tacrolimus Exposure Before and After a Switch From Twice-Daily Immediate-Release to Once-Daily Prolonged Release Tacrolimus: The ENVARSWITCH Study. Transpl Int. 2023 Aug 1;36:11366. doi: 10.3389/ti.2023.11366
For more information:
| Tryptique de l’étude |
IMPAKT is a clinical study titled “Pharmacokinetic Study of Adoport® (Tacrolimus) in Patients With de novo Kidney Transplantation (IMPAKT)”.
The primary objective was to develop a population pharmacokinetic model for Adoport® in de novo kidney transplant recipients.
A total of 30 kidney transplant patients were enrolled across four transplantation centers in France.
IMPAKT was sponsored by the University Hospital of Limoges (CHU de Limoges) and received financial support from SANDOZ Laboratories.
The project, led by Prof. Pierre Marquet, was coordinated by Dr. Caroline Monchaud.
ClinicalTrial number: NCT03076151
Study results were published in: Marquet, P., Destère, A., Monchaud, C. et al. Clinical Pharmacokinetics and Bayesian Estimators for the Individual Dose Adjustment of a Generic Formulation of Tacrolimus in Adult Kidney Transplant Recipients. Clin Pharmacokinet 60, 611–622 (2021). https://doi.org/10.1007/s40262-020-00959-y
For more information:
| Tryptique de l’étude |
These two cohort studies include data from a total of 820 kidney transplant patients followed in French transplantation centers, with follow-up periods extending up to 10 years for the longest cases.
The studies pursued multiple objectives, including the description of immunosuppressive strategies, drug exposure variables, and the exploration of adverse events, quality of life, and patient adherence to treatments.
Both cohorts were managed by the University Hospital of Limoges (CHU de Limoges) and received academic financial support from the CHU de Limoges and the 2011 Interregional Hospital Clinical Research Program (PHRC interrégional 2011).
The projects, led by Prof. Pierre Marquet, were coordinated by Dr. Caroline Monchaud.
The results and publication references are presented in this document.